Welcome to the world of element compounds and mixtures! This comprehensive guide will provide you with the element compounds and mixtures worksheet answers you need to excel in your studies. We will delve into the definitions, differences, and properties of elements, compounds, and mixtures.
Additionally, we will explore the various types of mixtures and chemical compounds, as well as their real-world applications.
Throughout this guide, we will provide clear explanations, engaging examples, and a structured worksheet to help you solidify your understanding. So, get ready to embark on a journey that will enhance your knowledge of element compounds and mixtures.
Element, Compounds, and Mixtures
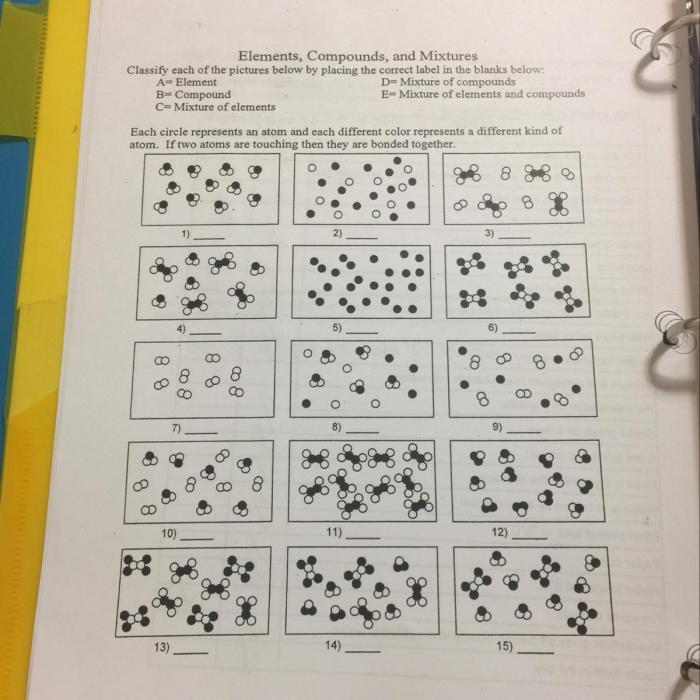
Elements, compounds, and mixtures are the fundamental building blocks of matter. Understanding their properties and characteristics is essential for comprehending the behavior of substances in the world around us.
Define and Explain:, Element compounds and mixtures worksheet answers
Element:An element is a pure substance that cannot be broken down into simpler substances by chemical means. Elements are represented by chemical symbols, such as H for hydrogen, O for oxygen, and Fe for iron.
Compound:A compound is a pure substance composed of two or more elements chemically combined in fixed proportions. Compounds have different properties from their constituent elements and can only be broken down into simpler substances by chemical reactions.
Mixture:A mixture is a combination of two or more substances that are not chemically combined. Mixtures can be separated into their constituent substances by physical means, such as filtration or distillation.
Types of Mixtures:
Homogeneous Mixtures:Homogeneous mixtures have a uniform composition throughout. They appear as a single phase and their components cannot be distinguished by the naked eye. Examples include salt water and air.
Heterogeneous Mixtures:Heterogeneous mixtures have a non-uniform composition. They appear as two or more distinct phases and their components can be distinguished by the naked eye. Examples include sand in water and oil in water.
Chemical Compounds:
Chemical Bonding:Chemical bonding is the process by which atoms are held together to form compounds. There are three main types of chemical bonds: ionic, covalent, and metallic.
Ionic Bonds:Ionic bonds form between atoms that have lost or gained electrons. The resulting ions are attracted to each other by electrostatic forces.
Covalent Bonds:Covalent bonds form between atoms that share electrons. The shared electrons are attracted to the nuclei of both atoms.
Metallic Bonds:Metallic bonds form between atoms of metals. The metal atoms share their valence electrons in a sea of electrons, which holds the atoms together.
Essential Questionnaire: Element Compounds And Mixtures Worksheet Answers
What is the difference between an element, compound, and mixture?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. A compound is a substance composed of two or more elements chemically combined in fixed proportions. A mixture is a combination of two or more substances that are not chemically combined and can be separated by physical means.
What are the different types of mixtures?
Mixtures can be classified as homogeneous or heterogeneous. Homogeneous mixtures are uniform throughout, while heterogeneous mixtures have visibly different components.
How are chemical compounds formed?
Chemical compounds are formed when atoms of different elements combine through chemical bonds. The type of bond formed depends on the electronegativity of the atoms involved.